ok, distilled is pretty good no doubt, people been using it for some time now.
deionized is what i meant to say. water is deionised by passing it through glass columns with deionising resins, and then it will contain no metal salts, acids, alkalis and other stuff which ionize's when dissolved in water.
ionization is measured by passing an electric current through the water with platinum electrodes to measure resistance. distilled water had a resistance of 58 thousand ohms per square centimetre of electrode at a distance of one centimetre apart. deionised water had a resistance of 4 million ohms.
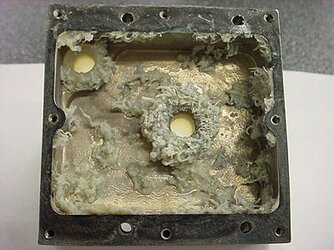
im no pro at water cooling comps but i been researching automotive stuff for years, and coolant system preservation has been one of the things ive read a lot about. when you do a search on water you realize that the earth really is covered in it, and so is google. lol
mainly what happens is when you bring metals in contact with each other they make a reaction. certain metals are ok together. aluminum and copper may not be in direct contact but thru the water the particles can contaminate surfaces, and the acids in the water also.
just like in a house if any part of your copper pipes are touching galvanized metal it creates a very low electric charge that causes corrision like what you would see on a battery terminal.
a lot of people (plumbers) will remove the old galvanized pipes and replace with copper to eliminate it. look on top of your hot water heater, you should see 2 die-electric unions, they break the contact between the 2 metals. one of the many things that people (plumbers) overlook is that even though there are no remaining pipes of galvanized in the house the pipes are still laying across a galvanized duct from the furnace.
so the pipes still corrode.
when you heat metals you create a faster environment for things to occur like that. as the molecules heat up they are moving faster and expand and seperate faster.
ok im tired. we can continue later.