New to the forums, have read quite a bit on here before but always ended up finding my answer, so I never signed up lol.
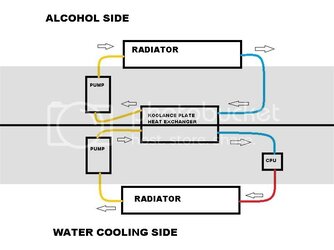
Anyways I have made a crude diagram of what I want to run, I know there are going to be some kinks to workout using the alcohol, like finding parts that wont be harmed by it and proper sealing of the system to fight evaporation. I would appreciate any thoughts or ideas on the subject.
Depending on the effectiveness of the heat exchanger will determine where it falls in the loop of course.
Thank you in advance.

Anyways I have made a crude diagram of what I want to run, I know there are going to be some kinks to workout using the alcohol, like finding parts that wont be harmed by it and proper sealing of the system to fight evaporation. I would appreciate any thoughts or ideas on the subject.
Depending on the effectiveness of the heat exchanger will determine where it falls in the loop of course.
Thank you in advance.
 to the forums!
to the forums!