- Joined
- May 1, 2004
- Location
- Bronx,newyork
Part 1, Part 2 with explanations will be posted at the end of this.
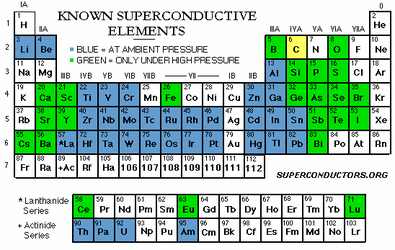
Hey all, Yuriman and me were talking on AIM the other day and got into a large fight (as usual) whether LN2 (liquid nitrogen 2, liquid nitrogen is diatomic always) would increase or decrease performance in cooling. Yuriman believed that Liquid nitrogen makes wires superconductors. I kept telling him that's impossible and here is why I am correct and he is wrong.
I basically set up an experiment with my Science teacher in school. Since she was going to show liquid nitrogen to the class I asked her if I may use some of it to run an experiment while showing the class that liquid nitrogen makes metal more resistive making bad conductors. She agreed.
So I set up my experiments, but I can't show you pictures right now because I don't have a digital camera. But, I do have this one paint picture/ diagram that may help you understand better.
This is basically is a diagram in what I did.

a= battery
b= containor fully of nitrogen
c= fan
d voltmeter
e= where i spliced.
I have got a 6 volt battery (one the big ones ;-)). I attached the alligator clips to it (part A), then connected a 12 volt care fan to the other side and stripped the wire in between so I could get my voltage measurements. With my voltage meter, I got my first reading which was 6.4 volts.
After getting my first measurements I put the middle of the wire into part B which held the liquid N2 and then waited a while, that way it could cool down. FYI this is -210C (Since it was in a cool container)
I then got my next reading of 6.2 volts. Clearly showing .2 losses in conductivity, even more so when I figured out that it want to be in the liquid nitrogen all the way, seeing we were running out. Knowing how elements behave
Reason for less performance, has to do with the element in this case copper and the electro activity of that element. It has to do with the electrons of the elements and as you slow down the electrons causes them to move slower causing decreasing performance. More will be added to soon…
most people think that liquid nitrogen makes metals super conductive. that is backwards the Ln2 makes magnets and magnets only super conductivity. magnets That is why when you go and get an M.R.I. done, that magnet is about $30,000 if not moreand is stored in liquid nitrogen. The colder a magnet gets the more conductive it becomes.
Hey all, Yuriman and me were talking on AIM the other day and got into a large fight (as usual) whether LN2 (liquid nitrogen 2, liquid nitrogen is diatomic always) would increase or decrease performance in cooling. Yuriman believed that Liquid nitrogen makes wires superconductors. I kept telling him that's impossible and here is why I am correct and he is wrong.
I basically set up an experiment with my Science teacher in school. Since she was going to show liquid nitrogen to the class I asked her if I may use some of it to run an experiment while showing the class that liquid nitrogen makes metal more resistive making bad conductors. She agreed.
So I set up my experiments, but I can't show you pictures right now because I don't have a digital camera. But, I do have this one paint picture/ diagram that may help you understand better.
This is basically is a diagram in what I did.
a= battery
b= containor fully of nitrogen
c= fan
d voltmeter
e= where i spliced.
I have got a 6 volt battery (one the big ones ;-)). I attached the alligator clips to it (part A), then connected a 12 volt care fan to the other side and stripped the wire in between so I could get my voltage measurements. With my voltage meter, I got my first reading which was 6.4 volts.
After getting my first measurements I put the middle of the wire into part B which held the liquid N2 and then waited a while, that way it could cool down. FYI this is -210C (Since it was in a cool container)
I then got my next reading of 6.2 volts. Clearly showing .2 losses in conductivity, even more so when I figured out that it want to be in the liquid nitrogen all the way, seeing we were running out. Knowing how elements behave
Reason for less performance, has to do with the element in this case copper and the electro activity of that element. It has to do with the electrons of the elements and as you slow down the electrons causes them to move slower causing decreasing performance. More will be added to soon…
most people think that liquid nitrogen makes metals super conductive. that is backwards the Ln2 makes magnets and magnets only super conductivity. magnets That is why when you go and get an M.R.I. done, that magnet is about $30,000 if not moreand is stored in liquid nitrogen. The colder a magnet gets the more conductive it becomes.
Last edited: